Abstract
Introduction Cold Agglutinin Disease (CAD) is a rare B-cell lymphoproliferative disorder of the bone marrow, manifested by autoimmune hemolytic anemia caused by binding of monoclonal IgM autoantibodies to I antigen. We have described the molecular background of CAD in the context of extensive clinical data, and found frequent somatic mutations in KMT2D and CARD11 genes and showed that CARD11 and CXCR4 gene mutations correlate with hemoglobin level. Moreover, we also have discovered trisomy 3 and 12 or 18 in CAD.
Gene expression data has not been reported in CAD and here we present novel RNA-seq data. Differential expression analysis revealed that the complement receptor 1 (CR1) gene was one of the genes most significantly down-regulated in CAD. Flow cytometry analysis further confirmed reduced CR1 protein expression on clonal CAD B cells. CR1 is an important negative regulator of B cell differentiation towards immunoglobulin-producing plasma cells and may thus contribute to CAD pathogenesis.
Materials for RNA sequencing We analyzed samples from 12 CAD patients collected for clinical trial (NTC02689986) and 4 healthy controls (HC). Clonal B cells from CAD patients and IGM+ memory B cells from HC, were procured using fluorescence-activated cell sorting of blood or bone marrow cells. IGM+ memory B-cells were used as controls in view of their shared CD19+/CD27+/IGM+ immunophenotype with clonal B cells in CAD.
Flow cytometry validation of CR1 expression CR1 protein expression was assessed by flow cytometry of a blood or bone marrow samples obtained from 15 CAD patients and blood samples from 5 HC. For each sample, CR1 expression was compared between IGM+ memory B-cells expressing immunoglobulin kappa light chain (IGK+) and IGM+ memory B-cells expressing immunoglobulin lambda light chain (IGL+), respectively. For CAD patients, the former had previously been demonstrated to be clonal cells, the latter functioned as non-clonal control cells.
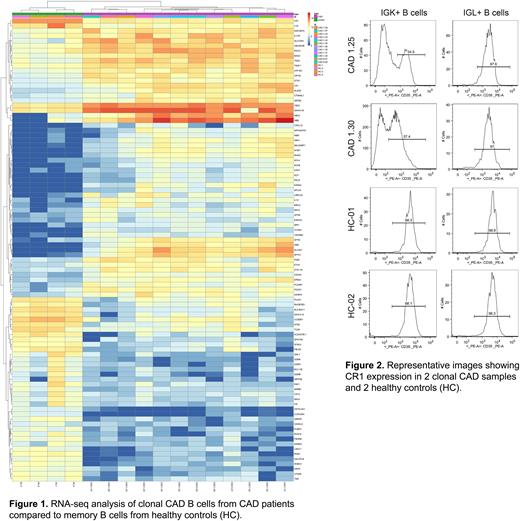
ResultsGene expression by RNA-seq RNA-seq data analysis and filtering resulted in a selection of 93 the most differentially expressed genes. The top 20 of the most differentially expressed genes from that list are: SLC4A1, SPTA1, YBX3, TESC, HBD, AHSP, TRAF1, HBA2, RHAG, CR1, CA1, SPTB, IL10, UBASH3B, ALAS2, HBA1, CRYM, RGCC, KANK2, IGHV4-34. These genes are upregulated at least 8 times in clonal CAD B cells compared to HC, except for CR1 which is downregulated ~11 times. The p-value, and p-value adjusted for multiple testing, are below 3.0-12 and 2.7-9, respectively, indicating statistically significant differences. Results are illustrated in Figure 1.
Validation of CR1 level by flow cytometry In CAD patients, CR1 expression in IGK+ B cells was significantly lower than in IGL+ B cells (Figure 2) as indicated by a median fluorescence intensity that was 10.8x lower in IGK+ than in IGL+ B-cells. HC samples showed similar levels of CR1 expression in IGK+ and IGL+ B cells, respectively. Samples from CAD patients usually showed a bimodal CR1 expression in IGK+ B cells, but not in IGL+ B cells. HC samples did not reveal heterogeneity of CR1 expression in IGM+ memory B cell populations.
Discussion CR1 is the receptor for C3b and C4b complement peptides (Klickstein et al. 1988), and its main function is to inhibit the complement cascade (Fearon 1979) and regulate proliferation of B cells (Khera et al. 2009). CR1 blocks proliferation induced by B-cell receptor activation, inhibits the differentiation of B cells to plasmablasts, and the production of immunoglobulins (Kremlitzka et al. 2012). CR1 ligation decreases phosphorylation of key molecules of the BCR-induced signaling cascade (Kremlitzka et al. 2016). Reduced expression of CR1 on B cells has previously been observed in several autoimmune conditions (Prokopec et al. 2010; Kremlitzka et al. 2016).
In conclusion, we demonstrated that CR1 expression is highly downregulated in clonal CAD B cells, both at the mRNA and the protein level. This may be an important finding in the context of CAD, because complement controls B and T cell responses, and regulates the adaptive immunity (Killick et al. 2018). Therefore, downregulation of CR1 in CAD might be responsible for increased proliferation, survival of autoreactive B cells and IgM autoantibody secretion. The demonstration of reduced CR1 expression by flow cytometry by IGK compared to IGL IGM+ memory B cells might also be exploited as a diagnostic test.
Disclosures
Berentsen:Anexon, Inc.: Consultancy; Apellis Pharmaceuticals, Inc.: Consultancy; Momenta Pharmaceuticals, Inc.: Consultancy; Sanofi Genzyme: Consultancy; SOBI: Consultancy; Jansssen-Cilag: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.